Clinical Data Management
Effectively manage your organization’s digital data with our tailor-made, high-tech solutions.
Clinical Data Management
Data management is the central requirement for enabling subsequent processes with the intent of providing structured data consistently for analysis and reporting. It is a critical phase in clinical research that leads to generation of high quality reliable and statistically proven data from clinical trials. Adapting innovative data management methodologies helps to ensure data integrity, quality and accountability in our process.
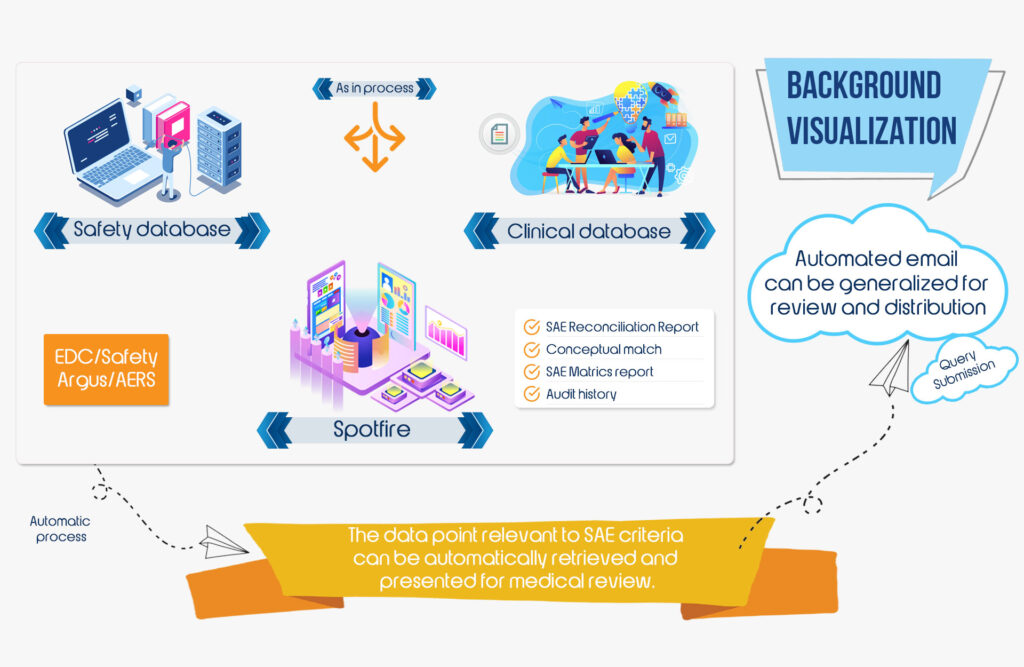
At Hyper Source, we have set principles and governance mechanisms in place to ensure the excellence of data with reduced turnaround time. We offer tailor made solutions to various procedures in clinical data management including Case Report Form (CRF) designing, CRF annotation, database designing, data entry, data validation, discrepancy management, medical coding, data extraction and database locking.
- Paper CRF /eCRF development and database design
- Data management plan
- Data entry, verification and validation
- Coding in MedDRA and WHO DD
- Query resolution and database locks
- Design safety database
- Electronic data integration
- SAE reconciliation
- Customized data listings
Contact Us
Contact Us
-
Hyper Source
5011 Southpark Drive, Suite 250, Durham NC 27713 - +1 (919) 244-4868
- support@hypersource.com