Pharmacovigilance
Subheading: Ensure compliance with regulatory agencies and expedite the development process.
Pharmacovigilance
Pharmacovigilance is a critical function in the life cycle of the drug focusing on patient safety and quality of life. While the principle of drug safety remains the same globally, the requirement of every regulatory authority is very specific and yet to be completely harmonized. Additionally, pharmaceutical organizations are challenged by the ever-changing regulatory requirements and unique needs of commercial partners.
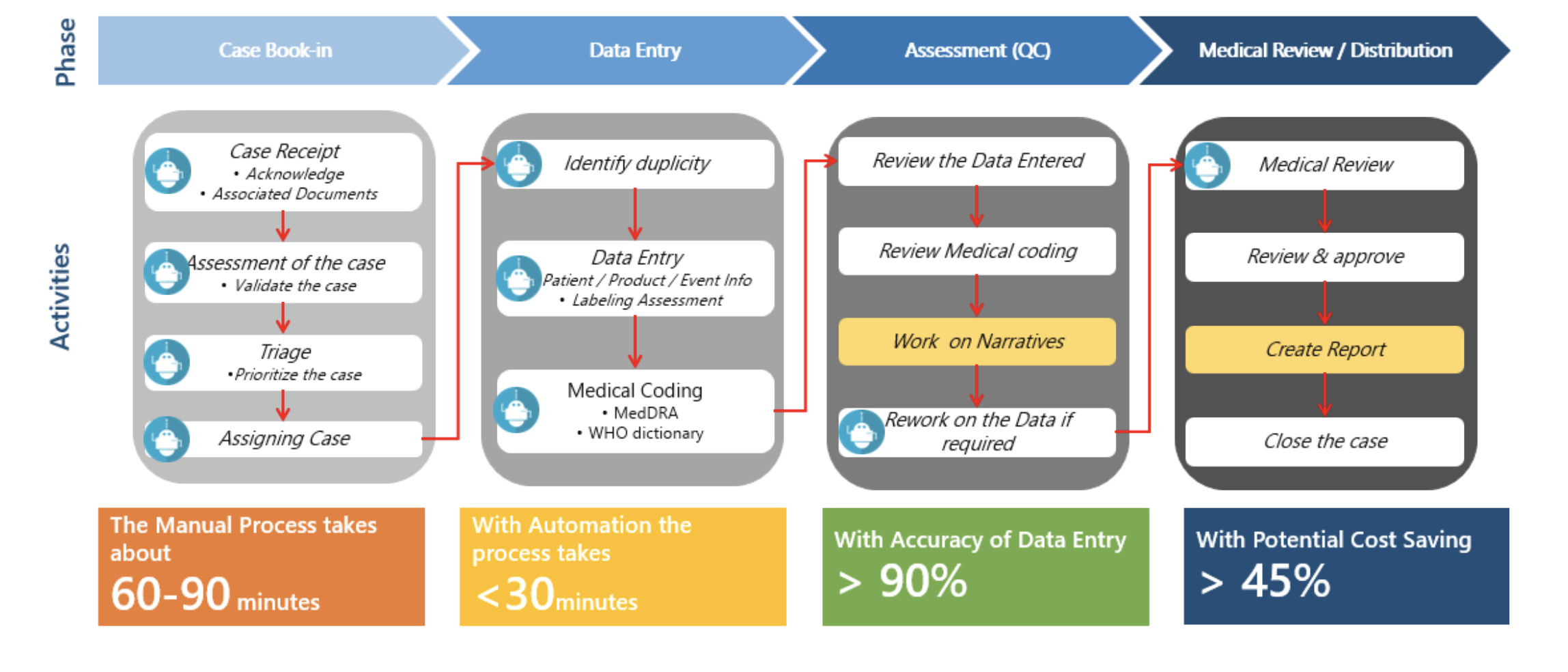
At Hyper Source, we focus on enabling our customers to deliver quality pharmaceuticals and medical devices with our expertise in risk minimization and maximizing patient safety. Our alignment to good pharmacovigilance practices benefits our customers in establishing confidence in healthcare professionals and patients across global markets.
- Medical information call center setup
- End-to-end case processing
- Aggregate reports
- Risk management plans
- Risk evaluation and mitigation strategies
- Literature screening and review
- Company core data sheet update signal detection and management.
Contact Us
Contact Us
-
Hyper Source
5011 Southpark Drive, Suite 250, Durham NC 27713 - +1 (919) 244-4868
- support@hypersource.com